Patients who suffer from cystic fibrosis have
been living longer and longer due to ever improving treatment options for the
disease, as explained in the post about cystic fibrosis. However, despite the best efforts of
researchers and physicians, patients will still frequently need a lung
transplant at some point in their life’s, because of the extensive damage
caused by the disease. This
procedure was not available for cystic fibrosis patients until about thirty
years ago1: this was evidently because of the increased risk of
infection in patients suffering from cystic fibrosis. As explained by Jens,
patients suffering from CF have an impaired innate immune system and a reduced
clearance of bacteria due to an altered innate immune response in the lung. While lung transplants prolong life expectancy of many patients, it still has its limitations. Transplanted lungs
generally don’t last very long, especially when a patient
suffers from frequent infections. The main complication in CF transplant
patients to date is infection with multi drug resistant bacteria, such as the
frequently occurring Burkholderia cepacian.1 Because CF causes damage to several organs
in the body, patients often have an extensive set of problems beyond the problems
in the lungs, which makes organ transplantation even harder and the patient
more vulnerable. The good news is that due to now quite extensive knowledge on
extrapulmonary complications in CF-patients, it is possible now to
transplant lungs to even severe cystic fibrosis patients even when they suffer from extensive
complications in other organs. Even though the procedure is now done quite
frequently in cystic fibrosis patients and has rapidly improved, it still
harbours a lot of risks. An example is the impaired immune function, which can have
great effect on the transplant success.4 Comorbodity has a great effect on transplant success too. Patients receiving transplants are often in a
later and more severe stage of the disease, which makes receiving successful transplants
and improved graft survival even more important.
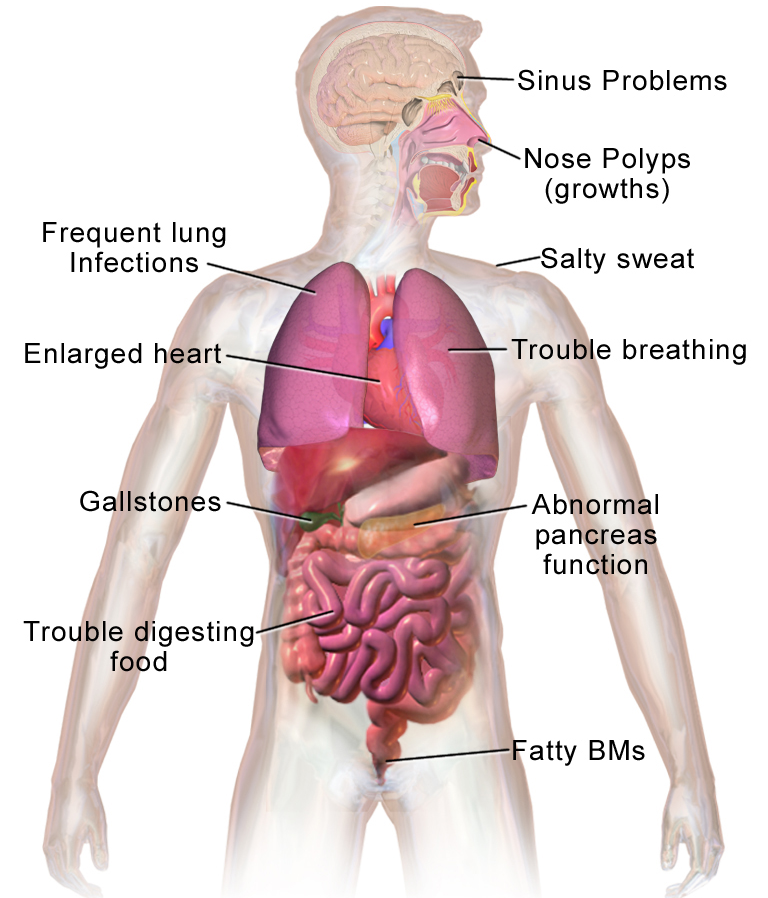
Figure 1 Possible additional
problems in Cystic fibrosis patients10
Are you curious as to how a lung transplant is
done? Below, there is video in which the entire procedure is explained:
Lung microbiome in survival rejection of
transplant
The lung microbiome is known to correlate with
survival in lung transplant recipients. In a study by Bernasconi et. al6,
they compared 112 patients post-transplant on long term graft survival
and the microbiome found in their lungs. The study showed that dysbiosis led to
more inflammation and unwanted graft remodelling which eventually led to a
worse prognosis. Furthermore, in a systemic review by Shasples et al.4 ,it is explained that infection and bronchiolitis obliterans are both key
players in long term graft survival. Bronchiolitis obliterans syndrome (BOS) is
known to be one of the most important limiting factor of long term survival in
lung transplant recipients. BOS is stimulated by graft rejection and in turn
graft rejection is induced by dysbiosis and inflammation. For example, the bacteria pseudomonas fluorescens was oftenly found in patients who did not
suffer from bronchiolitis obliterans syndrome, while Pseudomonas Aeruginosa seems to have an association with the
presence of bronchiolitis obliterans2. While we now start to
grasp how a a healthy microbiome is defined and what microorganisms are
generally found, we can start associate certain microorganisms and the degree of
diversity to better outcomes or rejection prognoses, and adapt maintenance
protocols in these patients accordingly. Since the lung has the highest rate of
acute and chronic rejection of any transplanted organ3 , it is
important to understand how the microbiota are influencing this and whether we could
influence the lung microbiota to reduce rejection and infection.
Infections and transplantation
A donor lung has been ridded of its defence
mechanisms, including the important cough reflex. Not only are the lungs in
constant contact with the outside world, they also are exposed to the colonized
native airway. Furthermore, in the donor lung there is no bronchial circulation
and lymphatic drainage.8 In addition to this already vulnerable state, the
patient will also be on immune suppressants to reduce the risk of graft
rejection. The failure and absence of the usual defence mechanisms lead to a heightened
rate of infection.8 Which, as mentioned before, impacts the graft
survival. The use of antibiotics used by the patient when they do acquire an
infection will also increase the chance of reinfection and reduce the diversity
of the lung microbiome which, as mentioned before, adds to the vulnerability, as was
also discussed in the post by Malak. Patients are the most at risk of infection within the first year after transplantation; more than 75% of infectious episodes occur within the first year.9 The
more infections a patient has, the higher the chance of graft rejection and
damage to the lungs. Thus, it is important to keep infections to a minimum!
Infections and lung microbiome in transplanted CF
patients
If you would compare cystic fibrosis patients post-transplant
with patients receiving a lung transplant for other reasons, the diversity of
bacteria in CF transplant patients seems to be less, which as I previously
pointed out, is correlated with a worse outcome and an increase of infections.7
While one could argue that the CF patients who experience these problems are now
in possession of a healthy pair of lungs, this does not completely solve the
problem. So why do CF patients still differ in lung microbiome from that of
other lung transplant recipients? While I could not exactly tell you why, we’ve
seen in the post by Malak about the lung- gut axis that there is more
influencing the lung microbiome than the lungs alone and CF patients also have
problems in other organs such as the intestines. Moreover these CF patients
still suffer from a somewhat impaired and sometimes even overactive immune
system. So we can conclude, that
for this group of patients a different approach is needed when it comes to a
healthy microbiome and acquiring infections. However, there is more known about the lung microbiome in cystic
fibrosis patients than that of the general population3, which begs
the question if we could use this information to influence the outcome of their
lung transplants. In other words, our work has been laid out for us if
it comes to lung transplants in patients with cystic fibrosis!
Written by Elise van Putten
Written by Elise van Putten
References:
1. Cecilia Chaparro & Shaf Keshavjee (2016)
Lung transplantation for cystic fibrosis: an update, Expert Review of
Respiratory Medicine, 10:12, 1269-1280, DOI:10.1080/17476348.2016.1261016
2. Robert P.
Dickson and John R. Erb-Downward (2014) Changes in the Lung Microbiome
following Lung Transplantation Include the Emergence of Two Distinct
Pseudomonas Species with Distinct Clinical Associations, PloS One, 9(5): e97214
3. Sushma K.CribbsJames and M.Beck (2017) Microbiome in the pathogenesis of cystic
fibrosis and lung transplant-related disease, Elsevier, Volume 179, January
2017, Pages 84-96
4. S.M. Palmer, L.H. Burch, A.J. Trindade, et
al. Innate immunity influences long-term outcomes after human lung transplant, Am
J Respir Crit Care Med, 171 (2005), pp. 780-785
5. L.D. Sharples, K. McNeil, S. Stewart, J.
Wallwork, Risk factors for bronchiolitis obliterans: a systematic review of
recent publications, J Heart Lung Transplant, 21 (2002), pp. 271-281
6. Eric Bernasconi, Céline Pattaroni and Angela Koutsokera, Airway Microbiota
Determines Innate Cell Inflammatory or Tissue Remodeling Profiles in Lung
Transplantation, American Journal of Respiratory and Critical Care Medicine,
Vol. 194, No. 10, Nov 15, 2016
7. E.S. Charlson, J.M. Diamond, K. Bittinger,
et al., Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota
after lung transplant, Am J Respir Crit Care Med, 186 (2012), pp. 536-545
8. BURGUETE, S. R., MASELLI, D. J., FERNANDEZ,
J. F. and LEVINE, S. M. (2013), Lung transplant infection. Respirology, 18:
22–38. doi:10.1111/j.1440-1843.2012.02196.x
9. Speich R, van der Bij W.
Epidemiology and management of infections after lung transplantation. Clin.
Infect. Dis. 2001; 33(Suppl. 1): S58–65.
10. Blausen.com staff (2014). "Medical
gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2).
DOI:10.15347/wjm/2014.010. ISSN 2002-4436.